Hylexxa® Products
Hylexxa” is a group of dermal fillers based on cross-linked hyaluronic acid. They are sterile, biodegradable, and biocompatible with a non-animal origin (non-allergenic). These fillers contain lidocaine for improved comfort during application. Produced using NAMO technology, “Hylexxa” comprises four sub-brands, each designed for specific and exclusive applications. The usage and injection of each sub-brand are determined by specialized physicians based on consumer needs.

Hylexxa® Soft
Hylexxa Soft is recommended for addressing minor to moderate skin depressions due to its specific formulation, which provides suitable rheology and elasticity properties. It can be used in areas around the mouth, around the eyes, frown lines, nasolabial folds, under the eyes, as well as between the eyebrows.
Hylexxa® Medium
Hylexxa Medium is recommended for the correction and filling of moderate skin depressions due to its specific formulation, which provides suitable rheology and elasticity properties. It is designed for volumizing and addressing medium-depth wrinkles and lines, aiding in restoring volume and reducing moderate wrinkles. Therefore, it can be used in areas around the mouth and eyes, as well as for medium-volume augmentation, such as in the lips.


Hylexxa® Deep
Hylexxa Deep is recommended for the correction and filling of deep skin depressions due to its specific formulation, which provides suitable rheology and elasticity properties. It is designed to address and restore volume, diminishing deep wrinkles and lines. As such, it can be used in areas around the nose, smile lines, lip volumization, and sculpting the soft tissue in the marionette lines.
Hylexxa® Volume
Hylexxa Volume is recommended for facial soft tissue correction due to its specific formulation, providing suitable rheology and elasticity properties. It is recommended for volumizing the cheeks, chin, nose, and sculpting the face’s angles. Additionally, it can be used to address and diminish very deep wrinkles and lines.


A leader in providing innovative and forward-looking solutions to improve a positive attitude to life
Instruction:
Hylexxa® Soft is a sterile, non-pyrogenic, biocompatible, and biodegradable cross-linked hyaluronic acid gel, which is not of animal origin. The product contains Lidocaine, which helps to reduce pain during and after the injection.
Composition:
Cross-linked Hyaluronic Acid | 22 mg/1.1 ml |
Lidocaine Hydrochloride | 3.3 mg/1.1 ml |
Phosphate Buffer pH 7 q.s. | 1.1 ml |
It is presented in one single-use pre-filled syringe (PFS) sterilized by moist heat. Each package contains one instruction leaflet, one syringe, two traceability labels, and two disposable 30G1/2 needles sterilized by radiation.
Indications:
The product is an injectable implant for the filling of fine to moderate cutaneous wrinkles including:
Mode of Administration:
Hylexxa® is a filling gel injected into the subcutaneous or deep dermis layer by authorized physicians Who have appropriate training and experience in injection techniques for volume restoration.
Direction for assembly:
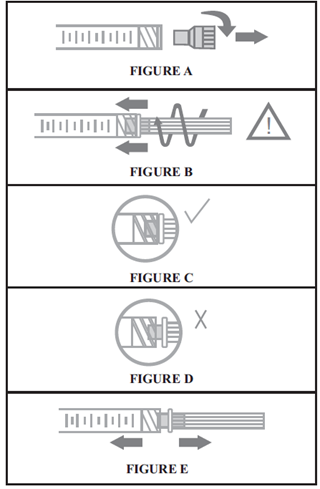
For optimal use of Hylexxa® products, it is essential that the needle is properly assembled to the syringe. See figure.
NOTE: If the position of the needle cap is as shown in Figure D, it is not attached correctly. Continue to tighten until the needle is seated in the proper position.
STEP 4: Remove the needle cap, and hold the syringe body in one hand and the needle cap in the other. Pull in opposite directions to remove the needle cap without twisting, as shown in Figure E.
Post-injection care:
Side effects:
These include, but are not limited to:
Patients must report inflammatory reactions which persist for more than one week, or any other side effect which develops to their physician as soon as possible.
Storage condition:
It should be stored in a dry and cool place, at a temperature below 30°C, out of the refrigerator and away from frost and heat. It should also be kept out of the reach of children.
Contra-Indications:
It should not be used:
Do not inject in cases of:
The product is intended for single use only. Do not reuse it.
Composition:
Cross-linked Hyaluronic Acid | 27.5 mg/1.1 ml
|
Lidocaine Hydrochloride | 3.3 mg/1.1 ml |
Phosphate Buffer pH 7 q.s. | 1.1 ml |
It is presented in a single-use pre-filled syringe (PFS) sterilized by moist heat. Each package contains one instruction leaflet, one PFS syringe, two traceability labels, and two disposable 27 G1/2 needles sterilized by radiation.
Indications:
The product is an injectable implant intended for volume restoration and filling of moderate cutaneous depression and wrinkles including:
Hylexxa® Deep is a sterile, non-pyrogenic, biocompatible, and biodegradable cross-linked hyaluronic acid gel that is not of animal origin. The product contains lidocaine, which helps to reduce pain during and after the injection.
Composition:
Cross-linked Hyaluronic Acid | 27.5 mg/1.1 ml
|
Lidocaine Hydrochloride | 3.3 mg/1.1 ml |
Phosphate Buffer pH 7 q.s. | 1.1 ml |
It is presented in a single-use pre-filled syringe (PFS) sterilized by moist heat. Each package contains one instruction leaflet, one PFS syringe, two traceability labels, and two disposable 27G1/2 needles sterilized by radiation.
Indications:
The product is an injectable implant intended for volume restoration and filling of cutaneous depression and deep facial wrinkles including:
Composition:
Cross-linked Hyaluronic Acid | 27.5 mg/1.1 ml
|
Lidocaine Hydrochloride | 3.3 mg/1.1 ml |
Phosphate Buffer pH 7 q.s. | 1.1 ml |
Hylexxa® Volume is presented in one single-use pre-filled syringe (PFS) sterilized by moist heat. Each package contains one instruction leaflet, one PFS syringe, two traceability labels, and two disposable 27G1/2 needles sterilized by radiation.
Indications:
روش استفاده:
نحوه قرارگیری سر سوزن بر روی سرنگ:
مرحله 1: مطابق شکل (A)، درپوش سرنگ را خارج کنید.
مرحله 2: مطا بق شکل (B)، بعد از وارد کردن سوزن به درون سرنگ، بدنه سرنگ را نگه داشته، انتهای سوزن را محکم در انتهای LUER-LOCK سرنگ قرار داده و سوزن را با چرخاندن آن در جهت عقربه های ساعت محکم کنید تا در موقعیت مناسب قرار گیرد. (مانند شکل C)
توجه: چنانچه درپوش سوزن مطابق شکل (D) به درستی قرار نگرفته، محکم کردن را ادامه دهید تا سوزن در موقعیت مناسب قرار گیرد.
مرحله 3: مطابق شکل (E)، بدنه سرنگ را در یک دست و درپوش سوزن را در دست دیگر نگه دارید. بدون چرخاندن، سرنگ را در جهت مخالف کشیده و درپوش سوزن را خارج کنید.

مراقبت های بعد از تزریق:
عوارض جانبی:
– برخی از عوارض جانبی احتمالی ناشی از استفاده از این محصول که ممکن است فوراً یا با تأخیر رخ دهد، و بیمار باید از آن ها مطلع گردد شامل موارد زیر می باشد:
– درصورت بروزهرگونه واکنشهای التهابی (یا هر عارضه جانبی دیگری )، که بیش از یک هفته ادامه یابد، سریعا به پزشک اطلاع داده شود.
– هر گونه عوارض جانبی دیگر باید به نماینده یا توزیع کننده محصول گزارش شود.
شرایط نگهداری:
روش استفاده:
نحوه قرارگیری سر سوزن بر روی سرنگ:
مرحله 1: مطابق شکل (A)، درپوش سرنگ را خارج کنید.
مرحله 2: مطا بق شکل (B)، بعد از وارد کردن سوزن به درون سرنگ، بدنه سرنگ را نگه داشته، انتهای سوزن را محکم در انتهای LUER-LOCK سرنگ قرار داده و سوزن را با چرخاندن آن در جهت عقربه های ساعت محکم کنید تا در موقعیت مناسب قرار گیرد. (مانند شکل C)
توجه: چنانچه درپوش سوزن مطابق شکل (D) به درستی قرار نگرفته، محکم کردن را ادامه دهید تا سوزن در موقعیت مناسب قرار گیرد.
مرحله 3: مطابق شکل (E)، بدنه سرنگ را در یک دست و درپوش سوزن را در دست دیگر نگه دارید. بدون چرخاندن، سرنگ را در جهت مخالف کشیده و درپوش سوزن را خارج کنید.

مراقبت های بعد از تزریق:
عوارض جانبی:
– برخی از عوارض جانبی احتمالی ناشی از استفاده از این محصول که ممکن است فوراً یا با تأخیر رخ دهد، و بیمار باید از آن ها مطلع گردد شامل موارد زیر می باشد:
– درصورت بروزهرگونه واکنشهای التهابی (یا هر عارضه جانبی دیگری )، که بیش از یک هفته ادامه یابد، سریعا به پزشک اطلاع داده شود.
– هر گونه عوارض جانبی دیگر باید به نماینده یا توزیع کننده محصول گزارش شود.
شرایط نگهداری: