Syflexx®
Syflexx is a new generation of injectable visco-supplements for intra-articular use.
In Syflexx, in addition to its strong lubricating properties and durability, which are based on studies and an examination of the improvable aspects of existing products in the market, special attention has been paid to the design, formulation, and molecular weight of sodium hyaluronate based on biological characteristics and the mechanism of action, as well as the correlation of each with its effectiveness. Syflexx has various sub-brands in which elements such as steroids and biological stimulants are used in combination.


Syflexx® M
Syflexx M, due to its unique formulation, not only possesses lubricating properties and prevents joint surface wear but also has the highest adhesion to cell surface receptors. This adhesion stimulates fibroblasts biologically, leading to increased production of hyaluronans, collagen, and elastin, potentially contributing to the reconstruction of damaged joints. Additionally, its anti-inflammatory properties prevent the production of pro-inflammatory mediators, thereby reducing pain.
Syflexx® H
Syflexx H, owing to its specific formulation, contains the most effective viscosity and volume from high molecular weight sodium hyaluronate (HMW). This results in significantly enhanced lubricating properties, providing comprehensive joint surface coverage. With a prolonged treatment duration, it prevents joint surface wear and damage to the cartilage. This not only prevents progressive inflammation but also restores the joint’s range of motion.


Syflexx® B
Syflexx B is a new generation of injectable intra-articular visco-supplements designed and manufactured in a modified-release format. In addition to creating the highest duration of lubricating properties, this product stimulates biological activity and facilitates the reconstruction of damaged joints through the controlled release of linear sodium hyaluronate with medium molecular weight (MMW).

A leader in providing innovative and forward-looking solutions to improve a positive attitude to life
Syflexx® M
(Sterile sodium hyaluronate for intra-articular injection containing lidocaine)
Syflexx® M is supplied in an autoclave-sterilized single-use prefilled glass syringe (PFS). Each package contains an instruction leaflet and a PFS syringe and a 21G needle tip.
Compounds
Sodium hyaluronate with average molecular weight: 62.5 mg
Lidocaine hydrochloride: 7.5 mg
Phosphate buffer (pH equal to 7): until reaching the volume of 2.5 ml
This product is a sterile, non-pyrogenic, transparent, viscoelastic, biocompatible and degradable gel for intra-articular injection that is biosynthetically produced and disposable. This product must be prescribed by a licensed physician.
This product is used in the following cases:
Symptomatic treatment of mild to moderate osteoarthritis (OA) of the knee or hip.
Symptomatic treatment of pain associated with mild to moderate osteoarthritis of the ankle, shoulder and elbow.
Treatment of pain after joint arthroscopy in the presence of osteoarthritis within three months after surgery is indicated.
Amount and method of consumption
This product is a single-dose injectable product that must be injected 3 times at one-week intervals during a six-month treatment period. The recommended dose is 2.5 ml (one syringe) for each knee, hip or shoulder joint. The amount of prescription for other joints is based on the diagnosis and opinion of a specialist doctor.
Mechanism of action
Hyaluronic acid is a natural part of synovial fluid in the body and acts as a lubricant for cartilage and ligaments and as a shock absorber in joints. It has been found that synovial fluid in arthritic joints has much lower viscosity and elasticity than healthy joints. Hence, injecting this product into the joint to restore viscosity and elasticity can reduce pain and improve joint range of motion. Also, in this product, by using hyaluronic acid with medium molecular weight, biological stimulation and increased proliferation of fibroblasts and collagen production are provided.
Instructions
Please inform your patient that:
Maintenance
It should be stored in a dry and cool place at a temperature below 30 degrees Celsius, outside the refrigerator and away from frost and heat. Also keep out of reach of children.
Prohibited Usage
Syflexx® should not be injected in case of allergy to hyaluronic acid and similar compounds, as well as allergy to other components of the product.
Warnings
Precautions
Side Effects
Most of the side effects reported in clinical studies for the treatment of osteoarthritis of the knee, hip, shoulder, and ankle are described as transient pain, swelling, or local stiffness at the injection site.
These side effects were of mild or moderate severity and only occasionally required treatment with analgesics or NSAIDs.
Other side effects may include headache, local itching and skin reactions at the injection site.
Any other side effects should be reported to the treating physician and representative of this product or to the local distributor of the product
Syflexx® H
(Sodium hyaluronate for intra-articular injection containing lidocaine)
Syflexx® H is supplied in an autoclave-sterilized single-use prefilled glass syringe (PFS). Each package contains an instruction leaflet and a PFS syringe and a 21G needle tip.
Compounds
High molecular weight sodium hyaluronate: 90 mg
Lidocaine hydrochloride: 9 mg
Phosphate buffer (pH equal to 7): until reaching a volume of 3 ml
This product is a sterile, non-pyrogenic, transparent, viscoelastic, biocompatible and degradable gel for intra-articular injection that is biosynthetically produced and disposable. This product must be prescribed by a licensed physician.
This product is used in the following cases:
Symptomatic treatment of mild to severe osteoarthritis (OA) of the knee or hip.
Symptomatic treatment of pain associated with mild to severe osteoarthritis of the ankle, shoulder and elbow.
Treatment of pain after joint arthroscopy in the presence of osteoarthritis within three months after surgery is indicated.
Amount and method of consumption
This product is an injectable and single-dose product that must be injected once every 4 to 6 months to achieve the best results. The recommended dose is 3 ml (one syringe) for each knee, hip or shoulder joint. The amount of prescription for other joints is based on the diagnosis and opinion of a specialist doctor.
Mechanism of action
Hyaluronic acid is a natural part of synovial fluid in the body and acts as a lubricant for cartilage and ligaments and as a shock absorber in joints.
It has been found that the synovial fluid in arthritic joints has much less viscosity and elasticity than healthy joints, therefore, injecting this product into the joint by restoring and restoring the viscosity and elasticity of the synovial fluid can reduce pain and increase the range of motion of the joint. improve
Syflexx® B
(Sterile hyaluronic acid for intra-articular injection containing lidocaine)
Syflexx® B is supplied in an autoclave-sterilized single-use prefilled glass syringe (PFS). Each package contains an instruction leaflet, a PFS syringe and a 21G needle tip.
Compounds
Bi-phasic hyaluronic acid (cross-linked hyaluronic acid plus linear sodium hyaluronate with medium molecular weight) 90 mg
Lidocaine hydrochloride: 9 mg
Phosphate buffer (pH equal to 7): until reaching a volume of 3 ml
This product is a sterile, non-pyrogenic, transparent, viscoelastic, biocompatible and degradable gel for intra-articular injection that is biosynthetically produced and disposable. This product must be prescribed by a licensed physician.
Uses
This product is used in the following cases:
Symptomatic treatment of mild to severe osteoarthritis (OA) of the knee or hip.
Symptomatic treatment of pain associated with mild to severe osteoarthritis of the ankle, shoulder and elbow.
Treatment of pain after joint arthroscopy in the presence of osteoarthritis within three months after surgery
Amount and method of consumption
This product is an injectable and single-dose product that must be injected once every 9 months to achieve the best results. The recommended dose is 3 ml (one syringe) for each knee, hip or shoulder joint. The amount of prescription for other joints is based on the diagnosis and opinion of a specialist doctor.
Mechanism of action
Hyaluronic acid is a natural part of synovial fluid in the body and acts as a lubricant for cartilage and ligaments and as a shock absorber in joints.
It has been found that synovial fluid in arthritic joints has much lower viscosity and elasticity than healthy joints.
Injecting this product into the joint can reduce pain and improve the range of motion of the joint by restoring and restoring the viscosity and elasticity of the synovial fluid.
Also, in this product, using high technology, sodium hyaluronate with medium molecular weight is slowly released, which causes the continuation of anti-inflammatory effects and biological stimulation, increasing the proliferation of fibroblasts and collagen production.
Mode of Administration:
Hylexxa® is a filling gel injected into the subcutaneous or deep dermis layer by authorized physicians Who have appropriate training and experience in injection techniques for volume restoration.
Direction for assembly:
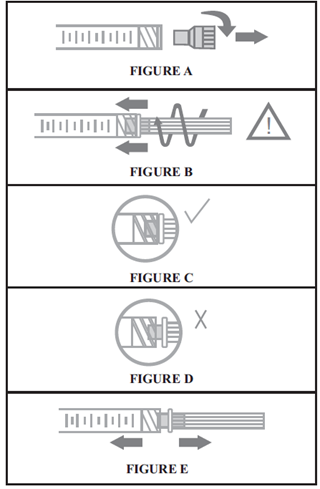
For optimal use of Hylexxa® products, it is essential that the needle is properly assembled to the syringe. See figure.
NOTE: If the position of the needle cap is as shown in Figure D, it is not attached correctly. Continue to tighten until the needle is seated in the proper position.
STEP 4: Remove the needle cap, and hold the syringe body in one hand and the needle cap in the other. Pull in opposite directions to remove the needle cap without twisting, as shown in Figure E.
Post-injection care:
Side effects:
These include, but are not limited to:
Patients must report inflammatory reactions which persist for more than one week, or any other side effect which develops to their physician as soon as possible.
Storage condition:
It should be stored in a dry and cool place, at a temperature below 30°C, out of the refrigerator and away from frost and heat. It should also be kept out of the reach of children.
روش استفاده:
نحوه قرارگیری سر سوزن بر روی سرنگ:
مرحله 1: مطابق شکل (A)، درپوش سرنگ را خارج کنید.
مرحله 2: مطا بق شکل (B)، بعد از وارد کردن سوزن به درون سرنگ، بدنه سرنگ را نگه داشته، انتهای سوزن را محکم در انتهای LUER-LOCK سرنگ قرار داده و سوزن را با چرخاندن آن در جهت عقربه های ساعت محکم کنید تا در موقعیت مناسب قرار گیرد. (مانند شکل C)
توجه: چنانچه درپوش سوزن مطابق شکل (D) به درستی قرار نگرفته، محکم کردن را ادامه دهید تا سوزن در موقعیت مناسب قرار گیرد.
مرحله 3: مطابق شکل (E)، بدنه سرنگ را در یک دست و درپوش سوزن را در دست دیگر نگه دارید. بدون چرخاندن، سرنگ را در جهت مخالف کشیده و درپوش سوزن را خارج کنید.

مراقبت های بعد از تزریق:
عوارض جانبی:
– برخی از عوارض جانبی احتمالی ناشی از استفاده از این محصول که ممکن است فوراً یا با تأخیر رخ دهد، و بیمار باید از آن ها مطلع گردد شامل موارد زیر می باشد:
– درصورت بروزهرگونه واکنشهای التهابی (یا هر عارضه جانبی دیگری )، که بیش از یک هفته ادامه یابد، سریعا به پزشک اطلاع داده شود.
– هر گونه عوارض جانبی دیگر باید به نماینده یا توزیع کننده محصول گزارش شود.
شرایط نگهداری:
روش استفاده:
نحوه قرارگیری سر سوزن بر روی سرنگ:
مرحله 1: مطابق شکل (A)، درپوش سرنگ را خارج کنید.
مرحله 2: مطا بق شکل (B)، بعد از وارد کردن سوزن به درون سرنگ، بدنه سرنگ را نگه داشته، انتهای سوزن را محکم در انتهای LUER-LOCK سرنگ قرار داده و سوزن را با چرخاندن آن در جهت عقربه های ساعت محکم کنید تا در موقعیت مناسب قرار گیرد. (مانند شکل C)
توجه: چنانچه درپوش سوزن مطابق شکل (D) به درستی قرار نگرفته، محکم کردن را ادامه دهید تا سوزن در موقعیت مناسب قرار گیرد.
مرحله 3: مطابق شکل (E)، بدنه سرنگ را در یک دست و درپوش سوزن را در دست دیگر نگه دارید. بدون چرخاندن، سرنگ را در جهت مخالف کشیده و درپوش سوزن را خارج کنید.

مراقبت های بعد از تزریق:
عوارض جانبی:
– برخی از عوارض جانبی احتمالی ناشی از استفاده از این محصول که ممکن است فوراً یا با تأخیر رخ دهد، و بیمار باید از آن ها مطلع گردد شامل موارد زیر می باشد:
– درصورت بروزهرگونه واکنشهای التهابی (یا هر عارضه جانبی دیگری )، که بیش از یک هفته ادامه یابد، سریعا به پزشک اطلاع داده شود.
– هر گونه عوارض جانبی دیگر باید به نماینده یا توزیع کننده محصول گزارش شود.
شرایط نگهداری: